Research in our lab covers a broad range of topics unified by a
single theme: the evolution of protein synthesis. Through
experimental evolution, bioinformatics, biophysics, and structural
biology we study protein synthesis machinery in different species to
understand how evolution happens in the world that Darwin never saw
– the world of proteins and nucleic acids. Currently, our lab is
developing three converging research directions.
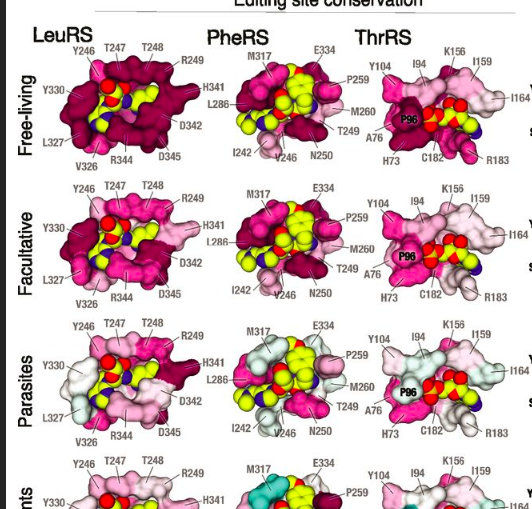
1. Adaptation to lifestyles
(Parasitism & genome evolution)
The impact of parasitic lifestyles on the evolution of proteins
and nucleic acids.
Lifestyles define the way we evolve. In the microbial world, the
transition from free-living to host-dependent lifestyles (symbiosis
or parasitism) causes a dramatic reduction in genome size; in
extreme cases, strictly host-dependent organisms may have genomes
that are 30-times smaller than the average genome of a free-living
species. By studying the protein synthesis machinery of organisms
with drastically reduced genomes, we are able to uncover the
fundamental principles of how the transition from a free-living
lifestyle to parasitism or symbiosis affects the folding, structure,
activity, stability, and evolution of proteins and nucleic acids in
the cell.
2. Adaptation to drugs
(How we can prevent or reverse drug resistance)
Combating drug resistance through experimental evolution.
When agriculture underwent a major crisis in the form of pesticide resistance in the 1970s, scientists and the public developed the philosophy of Integrated Pest Management (IPM), which forever changed our approach to pest control. Instead of trying to “eradicate” every single pest, IPM advocated approaching pests as an evolutionary problem and “managing” pests at an acceptably low level, thereby reducing pesticide use, cutting costs, and minimizing the risks of pesticide resistance. The aim of our work is to help create a similar fundamental shift in the way we approach the therapeutic treatment of infectious diseases. In doing so, we aim to find out how – by approaching infectious disease as an evolutionary process – we can attenuate, prevent, or reverse the evolution of antibiotic resistance in microbial pathogens and cancerous cells.

3. Adaptation to new environments
(Protein synthesis at extreme conditions)
Understanding the origin of life through studies of protein
synthesis machinery.
Ever since the pioneering studies by Carl Woese in the late 1970s,
components of the protein synthesis machinery (including rRNA and
ribosomal proteins) have been extensively used as the primary tool
with which to explore evolutionary relationships between species.
Ribosomal RNA, for instance, has been used as a life’s timekeeper to
determine the evolutionary age of species and their location on thetree of life. We are currently working to show that –
through high-throughput structural and computational studies of ribosomal
components – we can learn how to “read” the information that is written within the structures of ribosomes. Our
ongoing project shows that by studying changes in ribosome structure
between species, we can accurately predict the optimal growth
conditions for a given species and gain insights into atmospheric
changes that occurred in the distant past.
